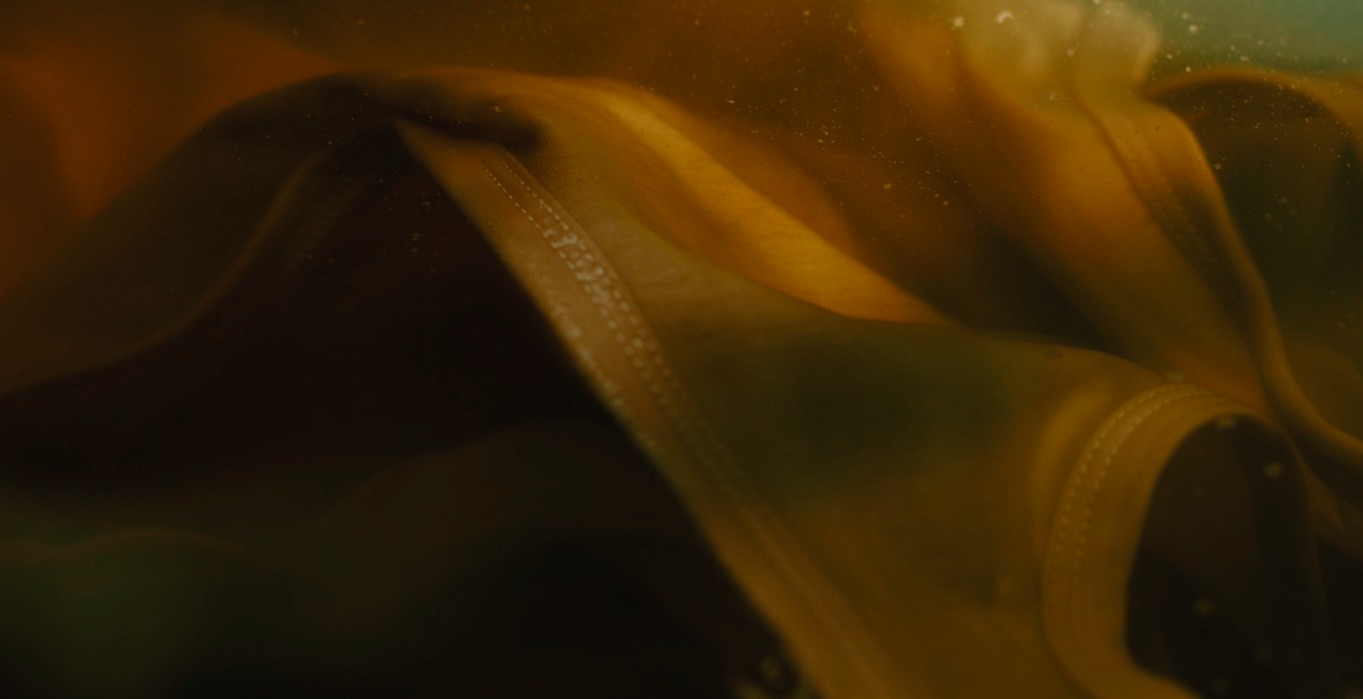Shifting Natural Dye | Ferrous Sulphate
The Process
To obtain certain colours, such as taupe, sage or grey, artisans adjust the natural colours of the tea dye with ferrous sulphate. After the iron solution is prepared, the fabric is submerged into the solution and the chemical reaction caused by the ferrous sulphate shifts the PH value of the dye to achieve the desired colour.
The colour changing process is carefully monitored as different factors could affect the time needed to achieve the correct colouration. Generally, fabric turns darker the longer it is left in the solution. Once the fabric has been adjusted to the desired colour, the iron molecules are rinsed off and the fabric is line dried.